- navigate_next Services
- navigate_next About SPILLO-PBSS
- navigate_next Experimental validations / Publications
FRONTIERS IN PHARMACOLOGY (2023) - Ben Toumia I, Bachetti T, Chekig-Ghedira L, Profumo A, Ponassi M, Di Domizio A, Izzotti A, Sciacca S, et al.
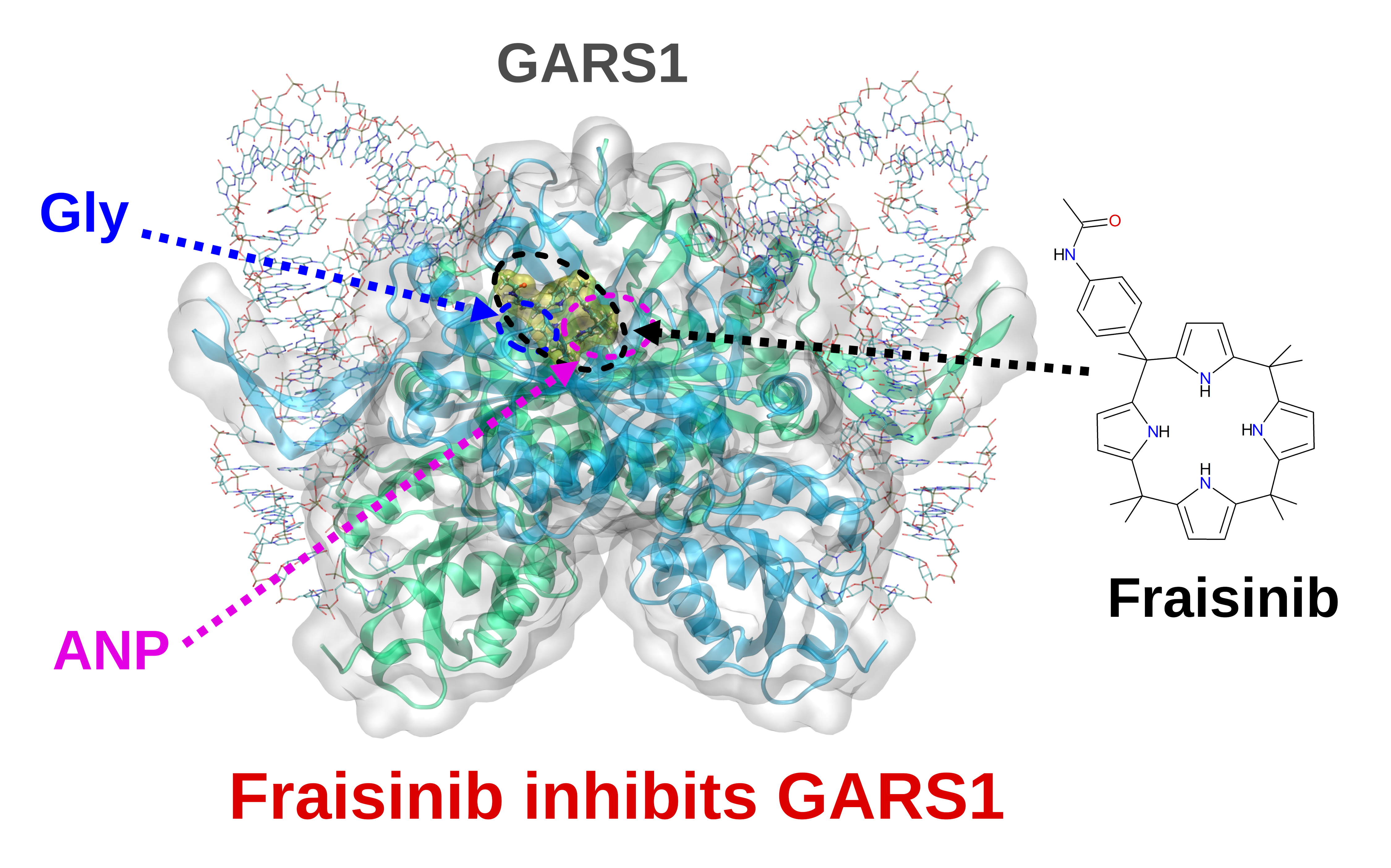
Fraisinib: a calixpyrrole derivative reducing A549 cells-derived NSCLC tumor in-vivo, acts as ligand of the Glycine-tRNA Synthase, a new molecular target in oncology
DOI: 10.3389/fphar.2023.1258108
Background and purpose: Lung cancer is the leading cause of death in both men and women, constituting a major public health problem worldwide. Non-small-cell lung cancer accounts for 85%–90% of all lung cancers. We propose a compound that successfully fights tumor growth in vivo by targeting the enzyme GARS1.
Experimental approach: We present an in-depth investigation of the mechanism through which Fraisinib [meso-(p-acetamidophenyl)-calix(4)pyrrole] affects the human lung adenocarcinoma A549 cell line. In a xenografted model of non-small-cell lung cancer, Fraisinib was found to reduce tumor mass volume without affecting the vital parameters or body weight of mice. Through a computational approach (SPILLOproject technology), we uncovered that glycyl-tRNA synthetase is its molecular target. Differential proteomics analysis further confirmed that pathways regulated by Fraisinib are consistent with glycyl-tRNA synthetase inhibition.
Key results: Fraisinib displays a strong anti-tumoral potential coupled with limited toxicity in mice. Glycyl-tRNA synthetase has been identified and validated as a protein target of this compound. By inhibiting GARS1, Fraisinib modulates different key biological processes involved in tumoral growth, aggressiveness, and invasiveness.
Conclusion and implications: The overall results indicate that Fraisinib is a powerful inhibitor of non-small-cell lung cancer growth by exerting its action on the enzyme GARS1 while displaying marginal toxicity in animal models. Together with the proven ability of this compound to cross the blood–brain barrier, we can assess that Fraisinib can kill two birds with one stone: targeting the primary tumor and its metastases “in one shot”. Taken together, we suggest that inhibiting GARS1 expression and/or GARS1 enzymatic activity may be innovative molecular targets for cancer treatment.
LINK to the FULL PAPER in FRONTIERS IN PHARMACOLOGY
